PharmaShots Weekly Snapshots (March 04 – March 08, 2024)
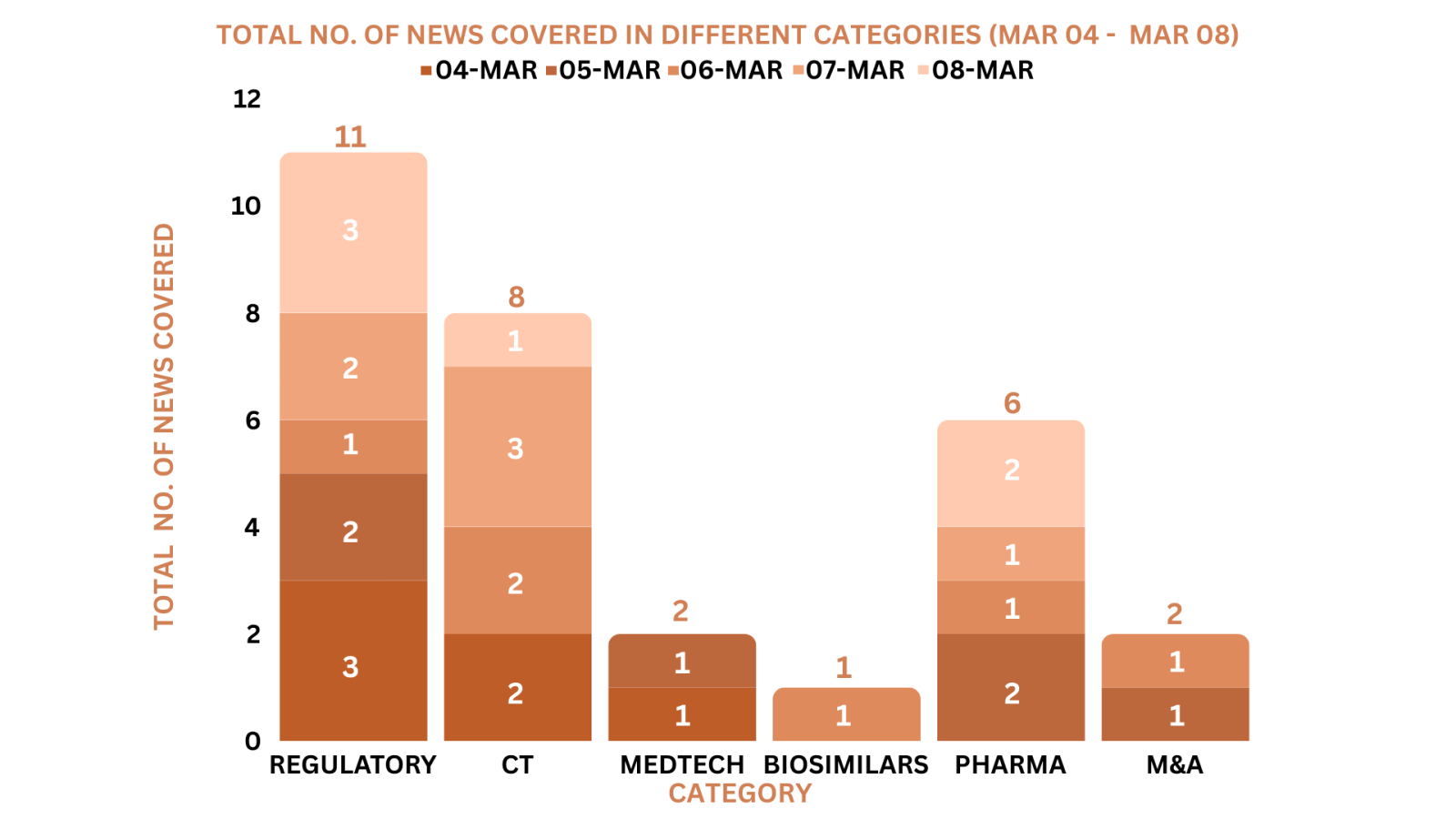
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials,Pharma, Animal Health & MedTech. Check out our full report below:

Cullinan Oncology Reports the US FDA’s IND Clearance of CLN-619 for the Treatment of R/R Multiple Myeloma
Read More: Cullinan
The US FDA Approves Johnson & Johnson’s Rybrevant in Combination with Chemotherapy as a 1L Treatment of NSCLC with EGFR Exon 20 Insertion Mutations
Read More: Johnson & Johnson
The EMA Validates two MAAs of AstraZeneca & Daiichi Sankyo’s Datopotamab Deruxtecan to Treat NSCLC and Breast Cancer
Read More: AstraZeneca & Daiichi Sankyo
The EMA Accepts for Review CymaBay’s MAA for Seladelpar to Treat Primary Biliary Cholangitis
Read More: CymaBay
The NMPA Accepts Innovent and AnHeart Therapeutics’ Second NDA for Taletrectinib to Treat Lung Cancer
Read More: Innovent & AnHeart Therapeutics
The EMA Validates Madrigal Pharmaceuticals’ MAA of Resmetirom for the Treatment of NASH/MASH with Liver Fibrosis
Read More: Madrigal Pharmaceuticals
Johnson & Johnson Submits Type II Variation Application to the EMA for Darzalex as a Treatment of Multiple Myeloma
Read More: Johnson & Johnson
The US FDA Approves Formosa Pharmaceuticals’ Clobetasol Propionate for Treating Post-Operative Inflammation and Pain Following Ocular Surgery
Read More: Formosa Pharmaceuticals
MindMed Receives the US FDA’s BTD for MM120 and Reports the P-IIb Trial Results for the Treatment of Generalized Anxiety Disorder (GAD)
Read More: MindMed
The US FDA Grants Accelerated Approval to BeiGene’s Brukinsa for the Treatment of Follicular Lymphoma (FL)
Read More: BeiGene
The US FDA Grants Approval to BMS’ Opdivo (nivolumab) in Combination with Cisplatin and Gemcitabine for the Treatment of Urothelial Carcinoma (UC)
Read More: BMS
Astellas Begins the P-III Study to Investigate Fezolinetant for the Treatment of VMS Associated with Menopause in Japan
Read More: Astellas
Novartis Reports Updated Results from the P-IIIb (SMART) Trial of Zolgensma for Treating Spinal Muscular Atrophy (SMA)
Read More: Novartis
Roche and Alnylam Report Data from the P-II (KARDIA-2) Study Evaluating Zilebesiran for the Treatment of Hypertension
Read More: Roche & Alnylam
CASI Pharmaceuticals and BioInvent Reports P-I Trial Results of BI-1206 to Treat Non-Hodgkin’s Lymphoma in China
Read More: CASI Pharmaceuticals & BioInvent
Abbott Highlights Data from the Real-World Studies of Freestyle Libre Systems and GLP-1 Medicines for Type 2 Diabetes at the 17th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD) 2024
Read More: Abbott
Gilead and Merck Highlight Data from the P-II Study Investigating the Combination of Islatravir & Lenacapavir for HIV
Read More: Gilead and Merck
GSK Reports Data from the P-III (DREAMM-8) Trial Evaluating Blenrep for the Treatment of R/R Multiple Myeloma (RRMM)
Read More: GSK
Tonix Pharmaceuticals Publishes Results of TNX-102 SL in P-III Trial for Military-Related PTSD in Psychiatry Research
Read More: Tonix Pharmaceuticals

The US FDA Approves Boston Scientific’s AGENT Drug-Coated Balloon for the Treatment of Coronary In-Stent Restenosis (ISR)
Read More: Boston Scientific
The US FDA Grants BDD to Quanterix’s Simoa P-Tau 217 Blood Test for the Diagnosis of Alzheimer’s Disease
Read More: Quanterix

Bayer and BridgeBio Join Forces to Commercialize Acoramidis for the Treatment of Transthyretin Amyloid Cardiomyopathy (ATTR-CM) in Europe
Read More: Bayer & BridgeBio
C4 Therapeutics and Merck KGaA Enter into a License & Collaboration Agreement to Discover Protein Degraders Against Oncogenic Proteins
Read More: C4 Therapeutics & Merck KGaA
Sonata Therapeutics Collaborates with the Champalimaud Foundation for the Development of SNT-3012 to Treat Pancreatic and Colorectal Cancers
Read More: Sonata Therapeutics
Merus and Gilead Partner for the Discovery of Antibody-Based Trispecific T-Cell Engagers
Read More: Merus & Gilead
Kyverna Therapeutics Signs a Collaboration Agreement with Stanford University to Evaluate KYV-101 for Multiple Sclerosis
Read More: Kyverna Therapeutics
Solid Biosciences Enters into an Exclusive License Agreement with Armatus Bio for AAV-SLB101 in Facioscapulohumeral Muscular Dystrophy (FSHD)
Read More: Solid Biosciences & Armatus Bio

Telix Acquires ARTMS along with its Advanced Isotope Production Platform
Read More: Telix & ARTMS
Peak Bio and Akari Therapeutics have Entered into a Merger Agreement to Form an Expanded Pipeline of Novel Antibody Drug Conjugate (ADC)
Read More: Peak Bio & Akari Therapeutics

The US FDA Approves Sandoz’ Wyost and Jubbonti, Biosimilar Versions of Xgeva and Prolia (denosumab)
Read More: Sandoz
Related Post: PharmaShots Weekly Snapshots (February 26 – March 01, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.